Changes in Anemone Species in Relation to Positioning and Microhabitats on the Rocky Shore
- Conor Kendrew
- Mar 27, 2015
- 9 min read
Introduction
The rocky shores which surround the inter-tidal zone in the UK host a great diversity
of invertebrate life, for many species this is the only habitat they inhibit. At low tides on the rocky shore it is also possible to see the beginnings of the submerged ecosystems of our coasts, these sightings are rare and allow you to see a group of organisms which seldom come into human contact. The reason for this biological diversity is due to the dynamic and complex abiotic factors on the shore supporting many niches of life within close proximity, this environment is outlined by Dipper et al., (1984).
Trevellas Cove's intertidal zone comprises of a mixed granite and slate geology, this location could be viewed as a typical Cornish rocky shore described by De La Beche, (1839) with the potential to support a variety of South West English organisms. The class Anthozoa (and more specifically coastal sea anemones) consists of many species which have become well adapted living in this habitat type, the speciation which has occurred between these rocky shore anemones may be a result of adapting to their differing niches. Although slow moving, anemones are a mobile organism capable of crossing the terrain along the shore in order to find the best suitable habitat. Physical variation between three species of anemone is apparent in figures 1-3, this could indicate that the organisms are exposed and adapted to differing selected living conditions. Therefore it would be a justifiable hypothesis that change in micro habitat has an effect of which species of anemone's are present on the rocky shore.

Figure 1: A Beadlet anemone (Actinia equina), the most common
species in the UK found frequently in rock pools, colours of this
species can vary from its most common red colour (displayed here)
or to a green/brown. The species retracts its tentacles when
not submerged. (Kendrew, 2015).

Figure 2: The Strawberry anemone (Actinia fragacea), structurally
similar to the Beadlet this anemone is generally larger than its
close relative. It also retracts its tentacles when exposed to the air,
this species is found only in its red colour with small green spots
on the column, from which it gains it's name. (Kendrew, 2015).

Figure 3: Snake-locks anemone (Anemonia viridis), this
species is more rare than those in figures 1 and 2. It often displays
a green coloured tentacle with pink tips, there is also a brown
coloured variation and the column of both is a brown colour.
Although it is possible to do so, the Snake-locks will not retract it's
tentacles when exposed to the air. (Kendrew, 2015).
Method
In order to get a fair representation of how varying species are positioned on the shore, a large and consistent number of anemones need to be recorded. To survey in this way, two groups carried out a S-shaped line survey across the shore from the low tide mark up to the point of the pebble beach, all anemones sighted are recorded and the search style must be as comprehensive as possible. The recordings are believed to have accounted for around 80% of the anemones on the shore with every aspect of the rocky shore habitat getting an equal amount of attention from the surveyors. This results in every microhabitat being searched equally. Many of the techniques and checks used to ensure legitimate data was collected are taken from the work of Sutherland, (2006).
When going out into the field it is important to understand the work which is required, and likewise how to mitigate risks which may arise. In order to maximise efficiency in working with a tidal field, the survey was arranged to start on the time of low tide, tides can be checked online at magicseaweed.com, (2015), if you forget to do this, beach lifeguards will also know the times. Four hours of surveying was allocated by the event organiser, this allowed careful work to take place over the unstable terrain without having to worry about rushing to collect data. Like for any other survey, a risk assessment was produced showing the group members what would be expected of them to ensure they remain safe during the survey. Maps of the survey area (figure 4) and recording sheets were prepared in order to make recording as simple as possible on the day.

Figure 4:Trevellas cove's Rocky shore shown here, this map shows the cove
exposed to a low tide. Digimap (2014)
Results
Results from the survey at Trevellas cove produced obvious trends, during recording, the differences between the micro habitats were clear and the anemone species generally fitted an independent niche along the shore (Figure 5). Only three species were found on the day, many more Strawberry (52) and Beadlet (92) anemones were found than the Snake locks (18), this was however expected.
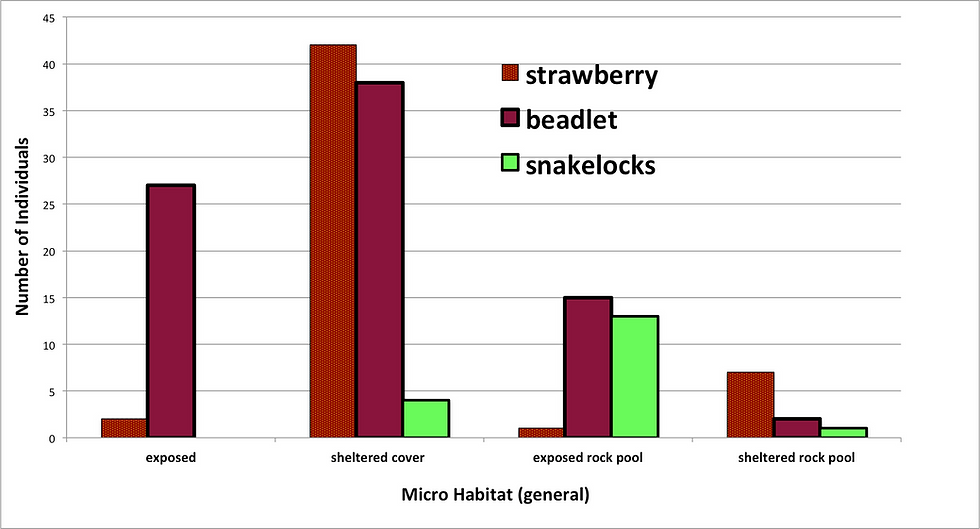
Figure 5: A graph showing the number of individuals of each species within four generalised habitats, group members discussed each habitats classification before recording.
A)
B)
C)
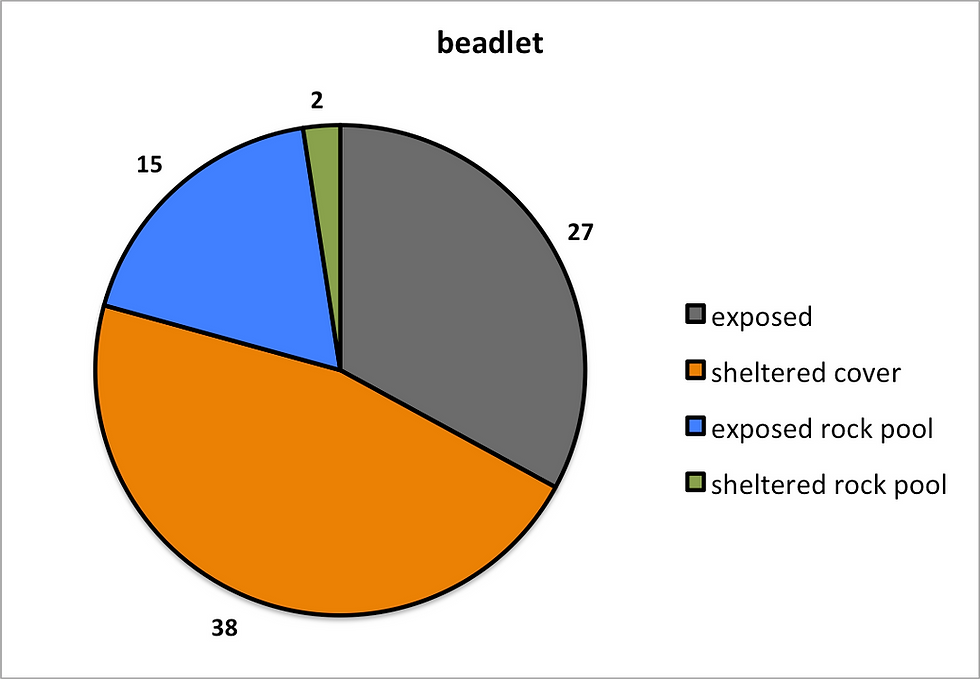
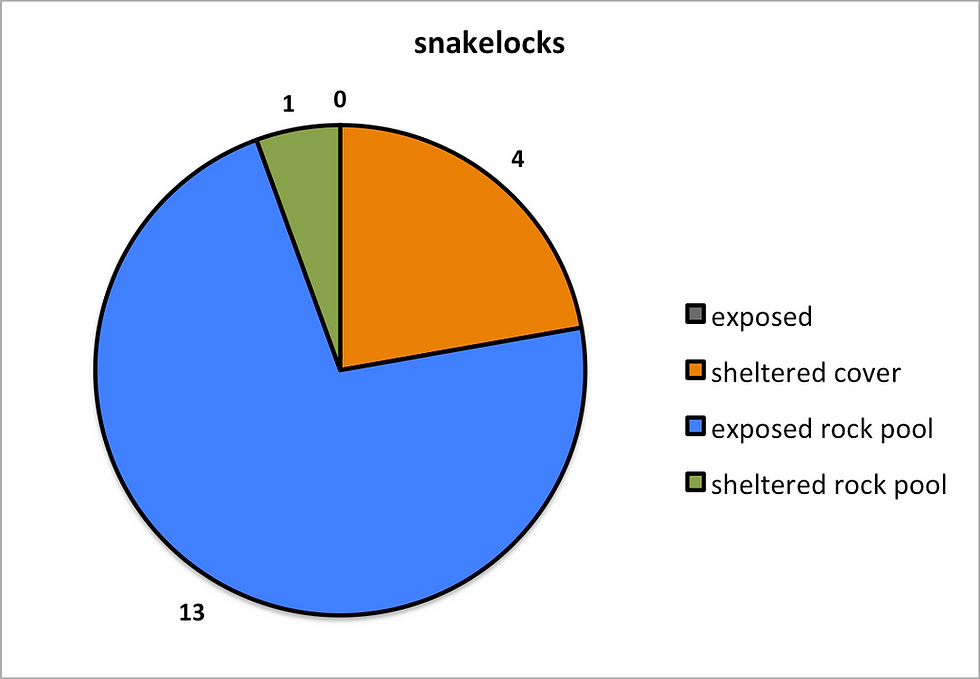
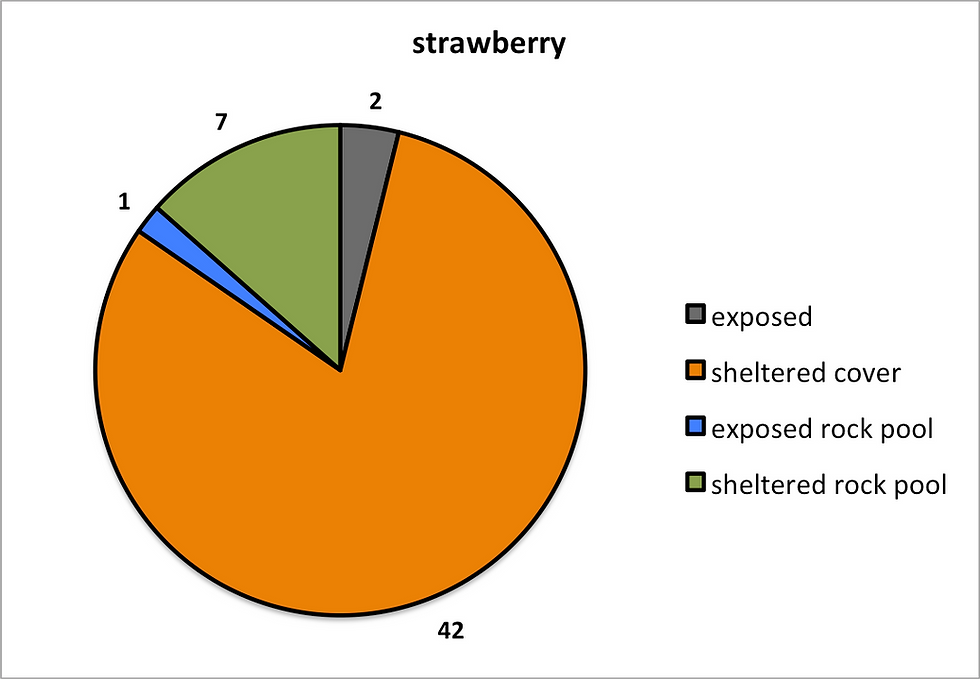
Figure 6: The results here demonstrate the preferences which
each species had in regards to micro-habitat. A) Snake locks B) Strawberry
C) Beadlet.
The results shown in figure 5 suggest that the differing species of anemone select differing micro habitats. The Beadlet anemone was the most common anemone and was represented across the most differing habitats (figure 6,C). The species was not opposed to exposure in rock pools or on bare surfaces, sheltered cover was represented by 33 individuals found under only one rock, which may be a result of other influences causing the species to select this habitat. Very few Beadlets were found in sheltered rock pools, however this habitat was not abundant at low tide.
Figures 5 and 6 suggest that the Strawberry anemone was the most habitat specific of the three species, opting to inhibit the low level shelter beneath rocks and under ledges. Its representation in sheltered rock pools would also suggest that this species is shelter loving/seeking.
The Snake-locks was the most illusive of the species, when found in sheltered cover it would be tucked into small spaces looking dry, however in its most common habitat of exposed rock pools the anemone would be submerged in small pools often in a group of a few individuals, this was a great difference from the other species which would almost always be found in a solitary position.
A)
B)
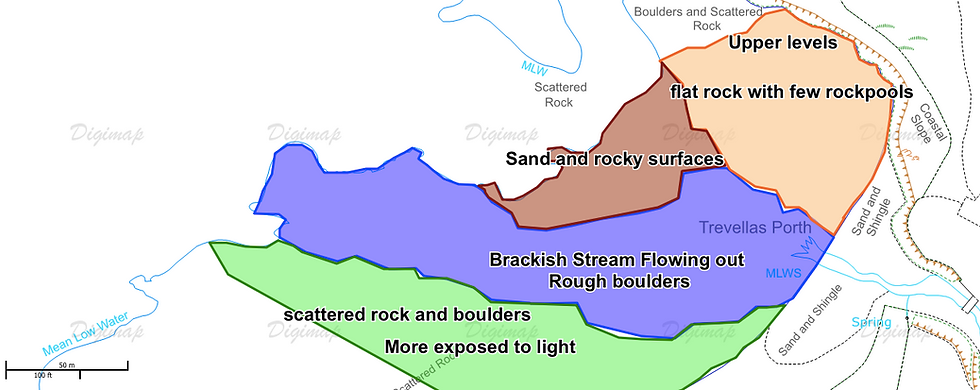
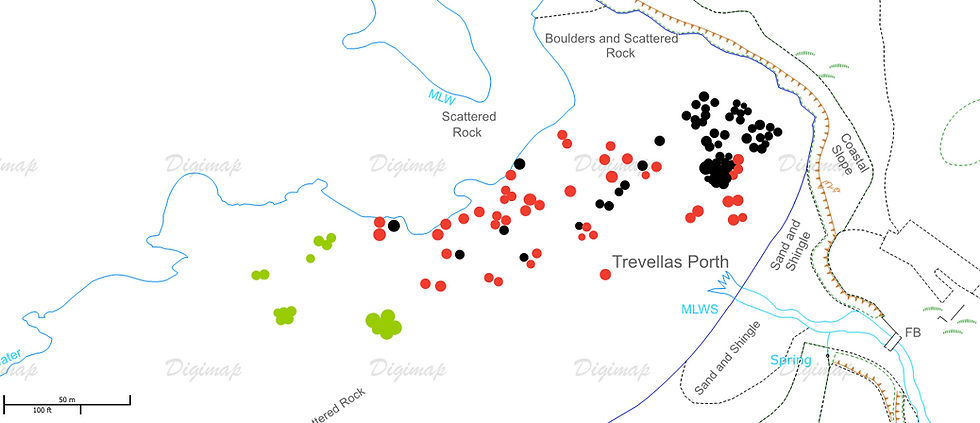
Figure 7: A) Map of anemones found across the shore, the species were located using a combination of GPS mapping and on-site map logging. Changes in circle size has no bearing on differences between individuals, Red: Strawberry anemone. Green: Snake-locks anemone. Black: Beadlet anemone. B) mapping of the general changes in dominant components of each area on the shore.
The trends shown in figure 7 present the bulk of Strawberry anemones at the lower shore in an area of damp, dense boulders. It is apparent that all the anemones were less populated at the area where the stream flows out, this location was more dominated by sea weeds such as bladder wrack and Kelp further down the shore. The Beadlets were much more prominent on he upper shore, which is exposed from the tide for long periods of time, it should also be noted that the 33 Beadlets located under the one rock pool were in this area. The Snake-locks anemone was found in groups along the section of the shore which had less shade from the surrounding cliffs and larger boulders.

Figure 8: It was a common occurrence that Snake-locks
anemones would be grouped together in rock pools.
(Kendrew, 2015).

Figure 9: This is the undercut boulder which held 33 Beadlet
anemones situated on the upper shore. (Kendrew, 2015).
Discussion
The Results From this survey show clear and strong trends which fit the predictions of the hypothesis, it is therefore apparent that different species are inhibiting different micro habitats. But what influences them to do this, and could there be external factors unrelated to micro habitats causing these results?
As shown, the Beadlet anemone is the most common anemone on the Trevellas shore and across the UK in general, this is due to its adaptation to a wide range of habitats and ability to grow and reproduce under many different conditions (Chomsky et, al. 2004). This species was found to inhibit areas at the lower shore as seen in figure 7, however the flat and exposed rocky surfaces of the upper shore is where this species was highly populated. Previous works on the areas colonised by Beadlets such as Brace and Quicke, (1986) would predict that the Beadlet should instead be populated across the rock pools of the shore and be highly populated at the low tide mark, the large number of Beadlets found under one sheltered rock on the upper shore also suggests that something is preventing the species from populating the lower shore, another factor is therefore changing the distribution of Beadlets at Trevellas.
The findings may be linked to the higher abundance of strawberry anemones at the lower levels, anemones are recorded to display aggressive territorial behaviour towards other individuals as they compete for space, they do this using their acrorhagi to reach across and sting neighbouring anemones, these blue tentacles can be seen in figure 1 on the right of the anemone. Studies by Turner et, al. (2003) have shown that during interspecific conflicts between these two species the Strawberry will often be successful. Strawberry anemones are the larger of the two species and it has become well understood that this anemone prefers shelter in the lower shore and has a lower range of habitat tolerance than its close relative, pointed out in a Cornish case study by Miceem, Waddell and Wilding, (2014). Strawberry anemones were once considered as a simple colour variation of the Beadlet until Carter & Thorpe (1981) reviewed the genetic differences of the two, the behavioural differences are clear along the Trevellas cove.
Factors restricting the Strawberry anemones distribution can be linked to habitat tolerance, for example exposure to dry sunlight or long durations of exposure from the tide. The Beadlet however is restricted by the competition from the strawberry anemone for the sheltered ledges at the lower tide range.
Differing From the two Actinia species there is the Snake-locks anemone. This species separates itself greatly from the other two found in this survey by fitting a very separate general niche. The majority of the Snake-locks were found in open rock pools of shallow depths on top of boulders or rocky outcrops, not a single one of these rock pools held another species of anemone, but there were a frequent amount of Snake-locks joined closely together forming a matt of tentacles. The species inhibits this habitat due to it's ability to feed and sustain through the photosynthesis of a symbiotic algae (Symbiodinum) living on the tentacles of the species, it therefore selects a habitat which offers enough light to justify photosynthesis and offers enough shelter from the open air (Bythell, et al. 1997). The anemone also reproduces in a way which differs greatly from the other two species, this organism has no medusa stage. During reproduction another polyp immediately forms in close proximity to another, this has resulted in the Snake-locks anemone being well adapted to sharing space with other individuals of the same species and is the reason so many were found next to each other in rock pools (Chintiroglou and Koukouras, 1992). The snake locks being situated at the more southerly section of the shore is predicted to be linked to more direct sunlight hours in this area, due to the shading of the cliffs being less prominent.
None of the anemone species were represented greatly in the Fresh waters of the stream outlet, particularly at the top of the shore, due to the obviously low tolerance of anemones to change in water composition. However Beadlets have been recorded to populate estuaries, Ager, (2001), these findings were not the case at Trevellas. A few strawberry anemones were the only organisms found here and they only occurred out of the waters flow under rocks.
Conclusion
The findings from the survey at Trevellas cove confirm that the three species present are in-fact filling differing ecological niches which results in species selecting different micro-habitats along the shore to maximise their productivity. Different behavioural and genetic traits which characterise these species are apparent and correlate with the conditions in which the individuals are situated. As with any field study, outliers and unpredicted specimens were found, but the general trend was strong across the shore allowing for a confident conclusion to be met. This study and many similar relevant resources are important in determining the characteristics of a group of taxa which were very under-studied in recent history.
References
Ager, O. (2001). "Actinia equina, Beadlet anemone. Marine Life Information Network: Biology and Sensitivity Key Information Sub-programme. Plymouth: Marine Biological Association of the United Kingdom"
Brace R. C. and Quicke D. L. J. (1986). Dynamics of Colonization by the Beadlet Anemone, Actinia Equina. Journal of the Marine Biological Association of the United Kingdom, 66, pp 21-47. doi:10.1017/S002531540003962X.
Digimap. (2014). OS Digimap Licence, updated December 2014. Alaviable at: http://digimap.edina.ac.uk/roam/os
Bythell, J. C., Douglas, A. E., Sharp, V. A., Searle, J. B., & Brown, B. E. (1997). Algal genotype and photoacclimatory responses of the symbiotic alga Symbiodiniumin natural populations of the sea anemone Anemonia viridis. Proceedings of the Royal Society of London. Series B: Biological Sciences, 264(1386), 1277-1282.
Chintiroglou, C., & Koukouras, A. (1992). A population of the sea anemone Anemonia viridis (Főrskal, 1775) and its associated flora and fauna, in the North Aegean Sea. Internationale Revue der gesamten Hydrobiologie und Hydrographie, 77(3), 483-495.
Chomsky, O., Kamenir, Y., Hyams, M., Dubinsky, Z., & Chadwick-Furman, N. E. (2004). Effects of temperature on growth rate and body size in the Mediterranean Sea anemone Actinia equina. Journal of experimental marine biology and ecology, 313(1), 63-73.
Dipper, F., et al. (1984). "Field Guide to Water Life of Britain". Readers digest; Nature lover's Library The readers digest association limited, London.
De La Beche, H. T. (1839). Report on the geology of Cornwall, Devon and West Somerset. Longman. pp. 177-178
Kendrew, C. (2015). Photo taken with iphone. Various Rock pools around Cornwall
magicseaweed: Perranporth (Droskyn) Surf Report, Surf Forecast and Live Webcams, (2015).
Avaliable at: http://magicseaweed.com/Perranporth-Droskyn-Surf-Report/166/. (Assesed: 2nd April 2015)
MCIEEM, M. G., Waddell, C., & Wilding, C. (2014). Intertidal Discovery Project–Coastal survey and mapping for conservation and public benefit in Cornwall. From the New Editor, 19.
Sutherlnd, W. J., (2006). Ecological Census Techniques, a handbook. Camberage University press, second edition.
Turner, V. L. G., Lynch, S. M., Paterson, L., León-Cortés, J. L., & Thorpe, J. P. (2003). Aggression as a function of genetic relatedness in the sea anemone Actinia equina (Anthozoa; Actiniaria). Marine Ecology Progress Series, 247, 85-92.


Comments